Pharma knowledge
Friday, 14 June 2019
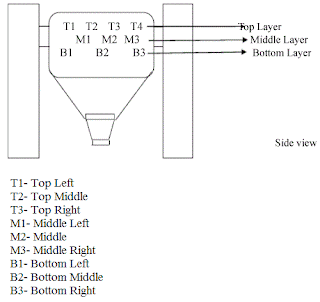
Light intensity Required in Pharmaceutical Industry
Light intensity in Pharmaceutical Industry
As comfort to work following are level for light Intensity.
1. Production Areas 200 lux light or more than it,
2. Visual inspection it should be between 300 lux to 600 lux
3. For documentation point of view or offices 650 lux to 700 lux.,
Sunday, 28 April 2019
FAQs on New Drug
What is a “new drug”?
A new drug means;
i. a drug, including
active pharmaceutical ingredient or phytopharmaceutical drug, which has
not been used
in the country
to any significant
extent has not
been approved as safe
and efficacious by
Central Licencing Authority
(CLA) i.e. DCG(I) with respect to
its claims; or
ii. a drug approved by the CLA
for certain claims and proposed to be marketed with modified or new claims
including indication, route of administration, dosage and dosage form; or
iii. a fixed dose combination of
two or more drugs, approved by CLA separately for certain claims and proposed
to be combined for the first time in a fixed ratio, or where the
ratio of ingredients
in an approved
combination is proposed
to be changed with certain claims
including indication, route of administration, dosage and dosage form; or
iv. a modified or
sustained release form of a drug or novel drug delivery system of any drug
approved by the Central Licencing Authority; or
v. a vaccine,
r-DNA derived product,
living modified organism,
monoclonal anti- body, stem cell
derived product, gene therapeutic product or xenografts, intended to be used as
drug;
What is a “subsequent new
drug”?
A subsequent new drug means a drug
approved by the Central Licencing Authority for certain claims
and proposed to
be marketed with
modified or new
claims including indication,
route of administration, dosage and dosage form. A subsequent new drug also
includes a new drug already approved in the country.
What is an “orphan drugs”?
An “orphan drug” means a drug intended to treat a
condition which affects not more than 5 lakh persons in India
Thursday, 7 March 2019
Root Cause Analysis With Example
Root
cause analysis is used in almost all industries to find out the cause of the
problems. It helps to improve the quality of product if it is used properly by
the management because it gives the way to implement the corrective and
preventive action.
Root
Cause Analysis is the most important factor in determining the quality of each
organization including the pharmaceutical industry, it is a methodology of
knowing the first action that leads to the sequence which in turn leads to the
problem and finds a way to solve this problem.
The
company auditors must observe the deviation and its relation with the past
events or intervention, they have to understand how could this intervention
cause the deviation. So, Root Cause Analysis is used to know the reason for
each deviation or bad results.
What are
the Types of Root Cause Analysis Techniques?
1. Group Thinking (also known as Brain
Storming): A group of experts meets together to find the
exact cause of the deviation and find a solution for it.
2. Fish bone Diagram (also known as cause and effect diagram): Fish bone diagram includes the potential cause of the problem and is
used in order to find the real cause.
3. 5 Whys:
5 whys tool works as its name, you
should ask yourself "why?" 5 times in order to get to the sequence
that causes the problem.
These 3
techniques will get you near the real cause, and it will help you to know what
are the changes that you have to do, where are your weak points and also how to
prevent the occurrence of this problem again.
Root
Cause Analysis consume a lot of time and resources, so it is only indicated
when you are very sure that an event has affected the organization badl and
needs an action to be done.
Examples
of Root Cause Analysis in Pharmaceuticals
Example 1:
A drug has
been doing badly
in the last
few weeks, the
Root Cause Analysis
revealed that the
cause is related
to non-optimum storage temperature which happened due to
starting of the summer.
The solution will be using refrigerators to
store this drug even while shipping it.
Example 2: A worker has chosen the wrong tool to join the
pipes together, the Root Cause Analysis revealed that the cause is the human error, but the problem actually originates from having bad
lighting to read the labels.
This means that learning this worker won't solve
the problem, the labels have to be clear and the lighting should be enough to
make him able to read them.
Example No.2 reveals the reason why deviation
investigation often fails if the organization inspectors did them. These
problems are:
1. A lot of Root Causes closed as just a human
error.
2. Incorrect or inappropriate use of the
techniques.
3. Corrective Action and Preventive Action is not pointing to
the real cause.
4. When
people interact with
the industry, the
human error is
a possibility, but
if the companies
did the necessary
measurements and used
the right techniques to detect
and prevent the human error, the human error would be nearly 5% only, this will
solve a lot of current and coming issues.
The
reason why there are some issues with root cause analysis is not the procedure
or the technique; it is mostly how the root cause analysis is being done. To prevent
this issue, just follow the corrective action and preventive action and it will
guarantee committed and satisfy inspectors.
Monday, 15 October 2018
Terms of Quality Risk Management
- Predictability: The ability to discover or determine the existence, presence or fact of a hazard.
- Harm: Damage to health, including the damage that can occur from loss of product quality or availability.
- Hazards: The potential source of harm.
- Risk: The combination of the probability of occurrence of harm and the severity of that harm.
- Risk Assessment: A systematic process of organizing information to support a risk decision to be made within a risk management process. It consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazard.
- Risk Initiation: Identifying the risk using suitable technique such as brain storming process.
- Risk Management: The systematic application of quality management policies, procedures and practices to the tasks of assessing, controlling, communicating and reviewing risks.
- Quality: The degree to which a set of inherent properties of a product, system or process fulfills requirements.
- Quality Risk Management: A systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across the product life-cycle.
- Quality System: The sum of all aspects of a system that implement quality policy and ensures that quality objectives are met.
- Risk Analysis: The estimation of the risk associated with the identified hazards.
- Risk Control: Actions implementing risk management decisions.
- Risk Mitigation: Systematic reduction in the extent of exposure to a risk and/or the likelihood of its occurrence. Also termed as risk reduction reduction.
- Risk Reduction: Actions taken to lessen the probability of occurrence of harm and the severity of that harm.
- Risk Evaluation: The comparison of the estimated risk to given risk criteria using a quantitative or qualitative scale to determine the significance of the risk.
- Risk identification: The systematic use of information to identify potential sources of harm (hazards) referring to the risk question or problem description.
- Risk Communication: The sharing of information about risk and risk management between the decision maker and other stakeholders.
- Risk Summary /Conclusion: It is summary report of observations / mitigation which has high RPN with appropriate actions proposed, target date and responsible person to reduce the identified risk.
- Risk Review Report: Review or monitoring of output/results of the risk management process considering (if appropriate) new knowledge and experience about the risk.
- Severity: A measure of the possible consequences of a hazard.
- High Risk – The equipment or system or process that will have focused and immediate impact on product quality. (Some of examples: Purified water system, Compression machine, metal detector system, non fill detector system on a packing line, on-line conductivity/Total Organic Carbon in a waters system line, Air handling Unit system for areas where product/materials are exposed during operation, power back up system sterility testing incubator, pure steam)
- Medium Risk – The equipment or system or process expected to have incidental or secondary impact on product quality. (Some of examples: raw water system, over coding machine)
- Low Risk – The non impact shall have no direct or indirect impact on product quality. (Some of examples: Fire alarm system, Effluent treatment plan incinerator, shrink wrapping machine).
- Effects: The results of an action or inaction (that result or could results from cause).
- Failure Mode: Different ways that a process or sub process can fail to provide the desired results.
- Failure Mode Effect Analysis (FMEA): A systematic method of identifying and preventing product process problem before they occur. It is primarily to evaluate a process prior to its implementation.
- Risk Priority Number (RPN): The risk priority Number or RPN is a numeric assessment of risk assigned to a process, or steps in a process as part of FMEA. Each failure mode gets a numeric score that quantifies likelihood of occurrence, likelihood of detection and severity of impact.
Tuesday, 9 October 2018
Difference between DPB and SPB
Two
types of pass boxes used in pharmaceutical industries:
1. Dynamic Pass
Boxes
2. Static Pass
Boxes
A) The SPB are used to transfer materials between two clean environments which are equally clean and
are designed to work with minimum personnel movement while DPB are used to pass materials from an uncontrolled environment to a
controlled environment. A SPB should never be used to transfer
material between a clean room and a non-clean room.
B) A DPB is a cubicle box which has interlocked doors on both sides. This protects the controlled
environment from being polluted while the transfer of a material is taking
place inside. A SPB, on the other hand, has got an electromagnetic
interlocking which is located between the two doors. The arrangement
is fitted with an LED indicator which helps to show whether any door is open.
C) A DPB has an
Interlock Guard which helps to control both the outlet and the inlet so that
there is no time that Both are opened. Due to this, dirt and other air born loose particles are removed from the materials being transferred to the manufacturing
area and vice - versa but in case of SPB, SPB doesn't have such a feature since it
only transfers material between two equally clean environments.
D) DPB is fitted with a suction
filter of around 0.3 microns that is a stainless steel product and a supply
filter of about 10 microns that is made of aluminium. A SPB doesn't
have the filters. The Dynamic Pass Box also has a pressure gauge ranging from 0
to 25 mmwc. It is also fitted with a motor blower of 1/5 hp for blowing out
dust particles. The SPB is designed as an air-lock device to
prevent ambient air from entering or clean air from disappearing from the clean
room.
E) For a DPB, the UV light's
specifications are 18 Watts while that of a Static Pass Box is 8 Watts. The
Ultraviolet light with hour mete is controlled by the interlocking arrangement
and it goes off when any of the doors open.
F) The Fluorescent lamp in a SPB is
about 40 Watts while that of a Dynamic Pass Box is about 20 Watts. The lamp is
controlled by the interlocking and when the doors are closed, the lamp is on.
Subscribe to:
Comments (Atom)
Featured post
-
Two types of pass boxes used in pharmaceutical industries: 1. Dynamic Pass Boxes 2. Static Pass Boxes A) The SPB are used to tran...
-
Question 1. How Many Tablets Shall Be Taken For Checking Friability? Answer : For tablets with unit mass equal or less than 65...